Advancing Innovation in Dermatology is pleased to make available our collection of scholar articles, industry news, and interviews with the professionals accelerating innovation in skin health and patient care. This content is yet another way beyond our in-person and virtual events to strengthen the community of innovators we aim to build and maintain.
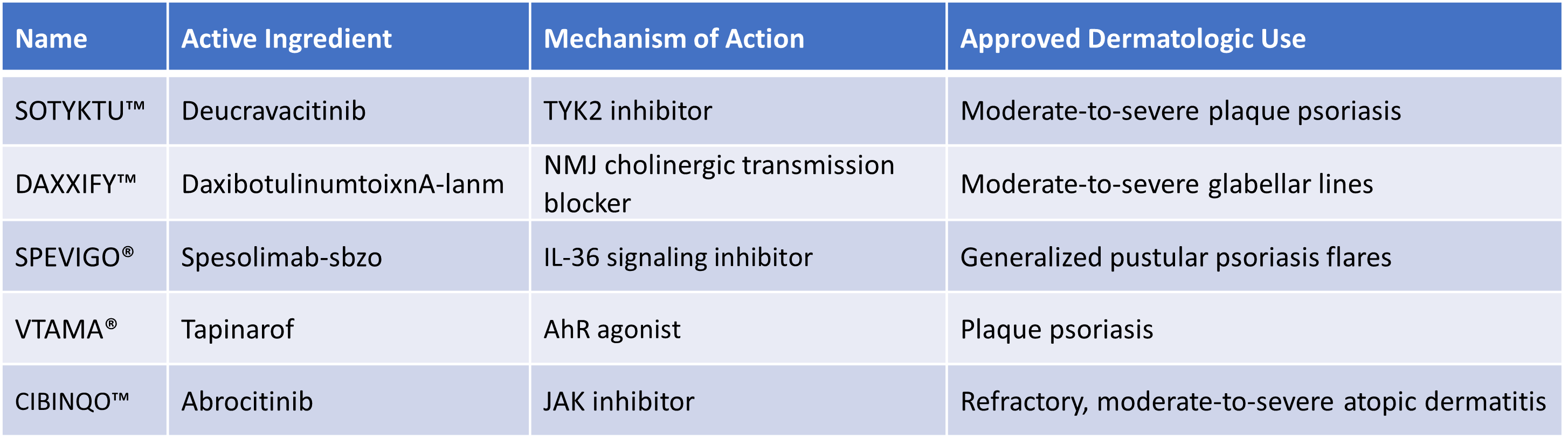
FDA and its Center for Drug Evaluation Research (CDER) approved 5 new molecular entities and therapeutic biological products this year. These are listed below (ordered from most recent to earliest).
These 5 approvals in 2022 compare to just 1 approval in 2021 and 3 approvals in 2020 by the FDA for skin diseases.
2021

2020
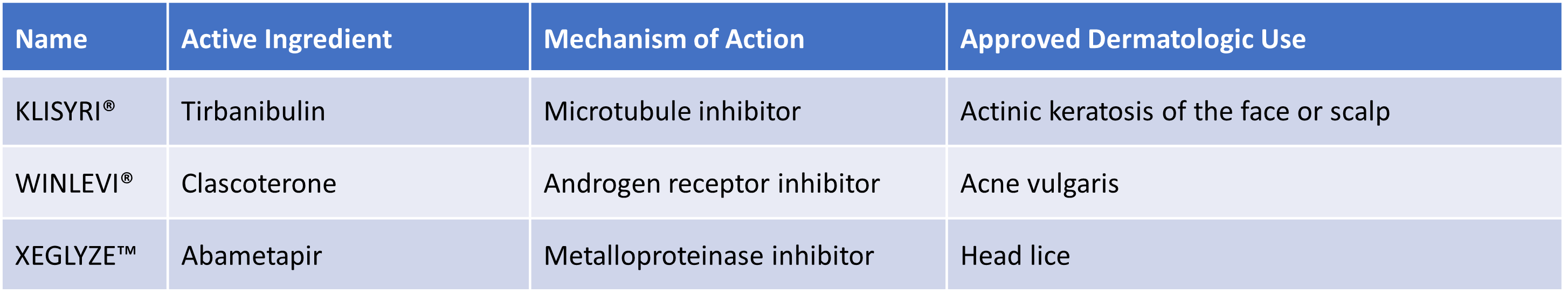
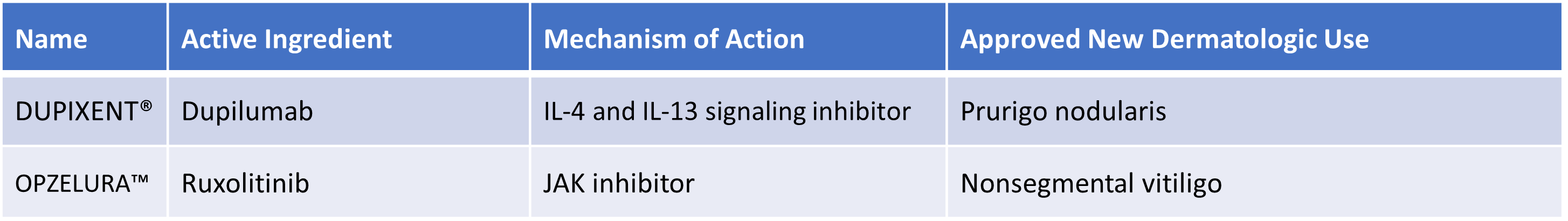
In addition, in 2022 there were new approvals for products for skin diseases, supplementing the use of active pharmaceutical ingredients previously approved for other indications or using other routes of delivery.
Two such therapeutics became the very first marketed products for treatment for dermatologic conditions.
In addition, OLUMIANT® (baricitinib), a Janus kinase inhibitor, became the first approved systemic medicine for adults with severe alopecia areata. ZORYVE™ (roflumilast), which is indicated for plaque psoriasis, is the first product to successfully formulate a phosphodiesterase 4 inhibitor for topical application to skin. EPSOLAY® (benzoyl peroxide) incorporates its active oxidizing agent with bactericidal and keratolytic effects into a novel microencapsulation cream and was approved for the treatment of inflammatory lesions of rosacea.
This year we witnessed substantial innovation in treating skin diseases, both in terms of the number of products approved by the FDA and the breadth of the conditions addressed. Research and development pipelines for dermatology indications are robust, and they are supported by scientific breakthroughs and by numerous stakeholders committed to investing in the field.
Many of these advances that can continue to impactfully improve skin health into the future will be presented and discussed at the Dermatology Summit taking place on January 8, 2023, in San Francisco around the JP Morgan Healthcare conference. Learn more about the Dermatology Summit.

Advancing Innovation in Dermatology Inc. is a nonprofit, tax-exempt charitable organization under Section 501(c)(3) of the Internal Revenue Code.
Donations are tax-deductible as allowed by law. | Privacy Policy | Cookie Policy
© 2025 Advancing Innovation in Dermatology Inc.. All Rights Reserved. | Web Design & Development time4design - Bucks County Web Design.